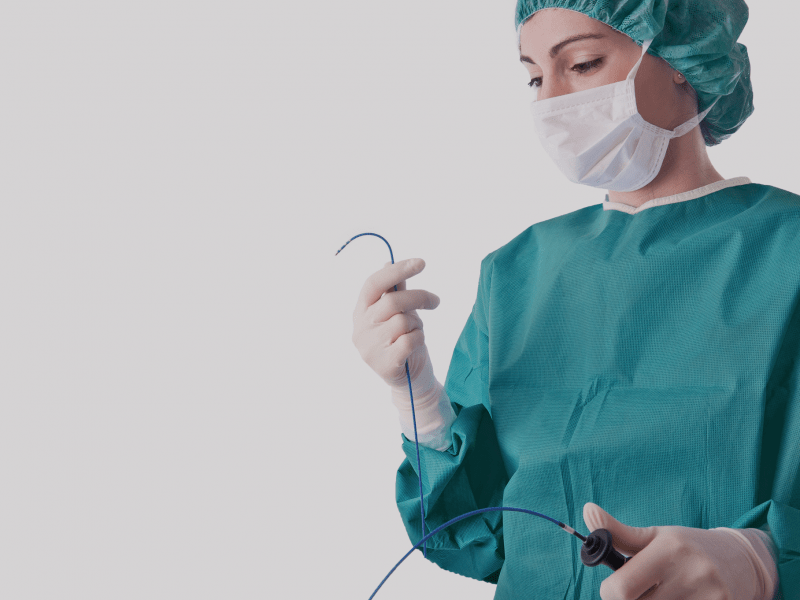
Electrophysiology devices
Vanguard has developed state of the art certified remanufacturing processes for more than 25 years. The remanufacturing of cooled ablation catheters and catheters with a 3D mapping function is one of our specialties.

CARTO3
Diagnostic and ablation catheters

EnSite Precision
Diagnostic and ablation catheters

More products
Discover our complete electrophysiology range
Vanguard sets the standard in medical remanufacturing of EP catheters
With the remanufacture of cooled ablation and 3D mapping catheters – Vanguard AG is taking medical remanufacturing of disposable medical products to a whole new level.
Vanguard: Experts in their field
The biggest challenge in remanufacturing cooled ablation catheters lies in the cleaning of the long and narrow lumen of the catheter shaft so that no residue remains.
When remanufacturing catheters with 3D mapping systems – the electronic memory chips and magnetic field sensors require special attention.
We make make no compromises with regard to safety or functionality. Our cleaning machines and our testing equipment are the product of many years of research and development and are tailored to the specific requirements of remanufacturing complex medical devices.
Our catheters are subjected to more than 20 individual tests. These include:
Unique rinsing concept
Potential residues in the lumen are flushed out by a specially developed and patented cleaning machine. In addition, there is continuous volumetric monitoring of the media flowing through the lumen.

Optical inspection
Visual inspection, for e.g. curvature shape or damage starting from the electrical connection to the tip, with up to 40x magnification.

Mechanical function test
Checking of all moving components as well as the components for controlling the catheter. In the case of a catheter with a force measurement function its complete function is also checked.

Vanguard Product Verification (VPV)
Testing of the entire electrical functionality such as insulation, continuity, capacitance and the temperature sensors by a specially developed testing system.
Microbiological Testing
Ensuring the effectiveness of the cleaning process by testing for protein residues on each catheter using the modificated OPA method, reference method according to ISO 15883 and the in-house L2 hygiene laboratory all including documentation.
