Medical Remanufacturing
Medical remanufacturing restores a used medical device to “as new” functional and safety standard with matching warranty. Medical remanufacturing enables a circular economy within the medical device sector. It helps conserve the planet’s valuable resources while keeping costs low — without compromising on safety or performance.
Rethink. Remanufacture.
Vanguard AG develops pioneering and certified medical remanufacturing processes that enable a circular economy for medical devices and allow hospitals to reduce carbon emissions, save resources and protect their budgets.

Sustainable
We extend the product life cycle of medical devices and enable a more sustainable future for the medical device sector. By giving new life to single-use medical devices, we combat climate change and protect our planet’s resources.
Economical
Through medical remanufacturing, the pursuit of sustainability pays off. There are no additional costs involved; in fact, medical remanufacturing can result in cost savings of up to 50 per cent.

Safe
Using certified, state-of-the-art processes, we set global standards for medical remanufacturing. We ensure that our remanufactured products are restored to “as new” functional and safety standards and fully comply with all EU regulatory requirements.
It’s possible with Vanguard! Original Equipment Re-Manufacturing.
With pioneering remanufacturing processes, Vanguard AG extends the product life cycle of single-use medical devices and thus enables a more sustainable healthcare system.

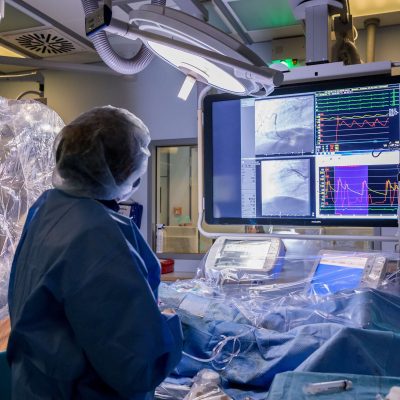
Positive Impact of medical remanufacturing on climate change
Fraunhofer UMSICHT recently completed a groundbreaking study that compared the resource efficiency and environmental impact of the production of a virgin catheter to a remanufactured catheter by Vanguard.
Our Purpose
We enable medical facilities to operate more sustainably, ensuring a better future for users, patients and the planet

Our Mission
We set global standards for medical remanufacturing that pave the way for a circular economy within the healthcare sector
Reducing CO2 emissions, conserving resources, saving costs
Original equipment manufacturers are increasingly looking for ways to become more ecologically and economically sustainable. We partner with OEM manufacturers to help develop suitable remanufacturing processes. Rely on the competence and professionalism of Vanguard for all your remanufacturing needs.
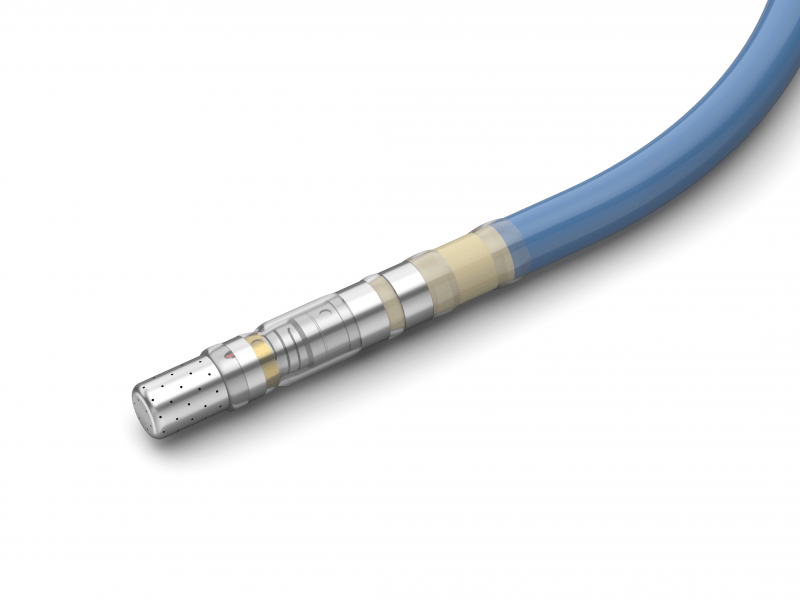
Products
Sustainability pays off
We offer a wide range of remanufactured electrophysiology and surgical devices.
Our devices meet the same criteria in terms of safety, functionality and performance as new devices. We assume product liability that matches that of the original manufacturer.
Vanguard News
Agroforestry Project Update: A Diverse Ecosystem Is Taking Shape on Field 701
A total of 36 woody vegetation strips have been established across the arable field. These are...
Why We Need to Talk About “Scope 4” and the Contribution Remanufacturing Can Make
A Missing Element in the Emissions Framework: The Concept of Scope 4 Scope 4 describes exactly...
ISO 13485 – Quality management as one of the prerequisites for safe medical devices
Why manufacturers of medical devices cannot do without this standard ISO 13485 is the...



